

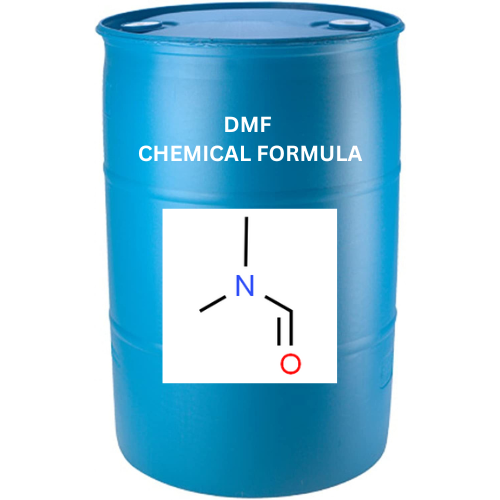
DMF is a document prepared by a pharmaceutical manufacturer and submitted solely at its discretion to the appropriate regulatory authority in the intended drug market.
Usage: Catalyst, electronic/ explosive/Resin Industry, paper chemical, Additive & Animal Nutrient
Composition and Information on ingredients